The past few years have been transformative for the open source hardware community, especially when it comes to health. What began as a response to crisis has grown into a deeper conversation about trust, documentation, and the future of open source medical and health devices, or as we call it Healthware. Here’s a look back at the first year of this journey, what we’ve learned, and where we’re headed next.
From Crisis to Concept: Covid and the Call for Openness
We all know that Covid changed the world. Before the pandemic, OSHWA had little involvement with medical devices with only a few projects in our certification directory related to health applications. However the urgency of the Covid crisis shifted everything. During Covid, we saw distributed manufacturing blossom with the new need for rapid and adaptable solutions to keep pace with the wake from this mysterious sickness. Examples of this in action was when the University of Wisconsin designed an open source facemask that Ford scaled into millions of units, and when Medtronic open-sourced their ventilator designs to meet urgent global demand.
After Covid, our community’s request began to shift, with two major desires making themselves known:
A medical-specific certification pathway that answered the question what does it mean to be both open source and a medical device?
A directory to connect the many groups already working in the space such as Field Ready, Ubora, Gila, and more.
In response to this shift in community needs, we began building what we now call the healthware ecosystem. To unify the field, we coined the term, healthware, to be a bridge between medical-grade devices (like pacemakers) and health-related devices (like step trackers).
An NSF POSE grant helped us formalize this work, and we launched over 100 interviews with researchers, makers, regulators, and industry stakeholders. From those conversations, a few key themes stood out:
Flexibility is essential. Certification must adapt to different geographies, regulations, and user needs.
Transparency matters. People want to know what their devices are doing, and they want equitable access to healthcare tools.
Liability is a concern. Many want to contribute but fear legal consequences if something goes wrong.
Standards bodies must be included. Collaborating with organizations like ISO and the FDA will be critical to long-term adoption.
Listening to the Community: Workshops & Takeaways
Our interviews fed directly into a series of four workshops—two virtual, two in-person. The message from the community was clear: they want a healthware certification.
Participants identified key stakeholders (makers, researchers, manufacturers, regulators, clinicians) and outlined what success might look like: alignment with local regulatory boards, strong documentation, replicability, and transparent governance. Importantly, participants emphasized that OSHWA’s role is not to guarantee safety, that’s for regulators, but to ensure openness, documentation, and community connection.
Trust, risk, and safety management emerged as recurring themes. People want OSHWA to steward this work in the near term, while leaving space for a potential international body in the future. Above all, clarity about what certification does and does not mean is essential.
When we synthesized all of the workshop discussions, four themes consistently surfaced:
Documentation – Seen as an essential ingredient for building safety, trust, and community in the open healthware ecosystem. Things such as verification and validation (V&V) records, 3rd party testing, and culturally adaptable documentation were all referenced as examples of desired documentation from the community. People also associated documentation with transparency and wanted to create a new norm of thorough documentation for the healthware community while it is still in its infancy.
Globality – Globality refers to the certification’s ability to reflect and be applicable to diverse global needs. Regulatory frameworks differ by country, cultural barriers affect adoption, and localized production hubs are critical. Ensuring that the certification process isn’t exclusively based on Western models of technological development to encourage global participation is essential.
Marketing – The open hardware movement at large needs people to be more educated about how it functions, and healthware is no different. Participants made it known that healthware needs more advocates, educational programming, and branding. Dispelling the myth of low cost equals low quality is paramount. Positive marketing successes for certification can encourage people to believe that healthware certification is a professional mark that communicates credibility and inspires trust.
Reproducibility – The ability to replicate designs, supported by robust documentation and testing, is a cornerstone of belief in healthware. Access to resources for verifying reproducibility and standardized methods for recreation are central to this goal.
Data Driven Insights & Challenges
At our workshops, participants shared their thoughts and reflections in real-time through collaborative documents, responding directly to prompts we provided. Afterward, our team carefully reviewed every contribution and scored them using a consensus-based approach called the Likert method. We focused on the four core themes – Documentation, Globality, Marketing, and Reproducibility – and looked at participant responses through three lenses: constraints, stakeholders, and vision. We also explored sub-themes such as Clarity, Transparency, Regulatory Compliance, Standardization, Validation, Interoperability, and Liability through these lenses as well.
The scoring system was as follows:
5 – Theme is directly named and emphasized repeatedly or with strong justification.
4 – Theme is clearly present and positively framed, but not central.
3 – Theme is implicitly mentioned or assumed, but not emphasized.
2 – Theme is weakly suggested or only incidental.
1 – Theme is absent or contradicted.
The results of the analysis are illustrated here:
Documentation
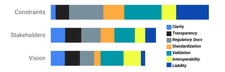
Globality
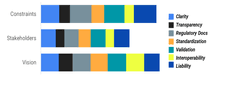
Marketing

Reproducibility
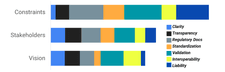
From our analysis, five unique insights emerged:
Regulatory & validation documentation is non-negotiable. It’s the strongest mechanism for building trust across geographies.
Liability fears are widespread. Contributors need clarity on responsibility and legal risk.
Interoperability is a tension point. Stakeholders value it, but it often emerges unevenly across contexts.
Marketing gaps are real. Open source remains poorly understood, requiring more education and awareness.
Reproducibility drives consensus. Standardization, clarity, and shared resources are seen as key to success.
Looking Ahead
This first year of the healthware journey has been about listening—through interviews, workshops, and careful synthesis. Moving forward, we’ll continue to:
Gather and analyze community feedback.
Develop educational materials, surveys, and content that make healthware accessible.
Build inclusionary on-ramps for global participation.
Clarify OSHWA’s role as a certifying and directory body—not a regulator.
The vision is clear: a thriving, global ecosystem of open healthware projects that are transparent, well-documented, and replicable. The journey is just beginning, but together, we’re building the future of open healthware.
